MOLECULAR AND SEROLOGICAL DETECTION OF CRIMEAN-CONGO HEMORRHAGIC FEVER VIRUS IN SULAIMANI PROVINCE, IRAQ

Tariq A.G.Aziz1, Dlovan J. Ali2,Dilshad O. Jaff3
1Dept. of Microbiology, College of Medicine, Sulaimani University-IRAQ
2 Dept. of Microbiology, College of Veterinary Medicine, Sulaimani University-IRAQ
3Gillings School of Global Public Health, University of North Carolina, Chapel Hill, USA
Email: [email protected]
Received **** 2016
Copyright © 2016 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/
Abstract
Background: Crimean-Congo hemorrhagic fever virus (CCHFV) is a member of the genus Nairovirus in the family Bunyaviridae and is transmitted by ticks of the Hyalomma genus. It causes severe disease in humans with mortality rates of 3-30%. In Iraq, the first case was reported in 1979; thereafter, 10 cases were reported and seven of these infected individuals died. Several cases were also reported in 1980 in Halabja city, Sulaimani province.
Methods: Blood samples were collected from two study populations, human and cattle, including from butchers working at a slaughterhouse. Ticks were also collected for virus detection from three villages in the Sharazoor district.
Results: Results of the Reverse Transcription-Polymerase Chain Reaction (RT-PCR) were negative for all human and cattle sera. Tissue prepared from ticks also was negative for CCHFV. An Enzyme-Linked Immunosorbent Assay (ELISA) technique showed that 30 (46.87%) of 64 human sera were positive for the anti-CCHFV IgG antibody.
Conclusion: The results showed that symptomatic Crimean-Congo hemorrhagic fever (CCHF) is an uncommon disease in the Sulaimani province and no clinical cases were reported because of
the eradication of ticks implemented by veterinary authorities. Other preventive approaches and strategies should be implemented and monitored regularly by the local authorities as well.
Keywords
CCHFV,CCHF, RT-PCR, ELISA
- Introduction (Heading 1)
Viral hemorrhagic fever (VHF) is a clinical illness that is becoming a serious threat to animals and humans.1 One of many VHFs is Crimean-Congo hemorrhagic fever (CCHF), an acute, highly contagious, tick-borne viral zoonotic disease that is listed as a notifiable disease.2 The disease is widely distributed globally with hemorrhagic manifestations and considerable mortality in humans but no specific clinical symptoms in animals.3,4 The disease is caused by Crimean-Congo hemorrhagic fever virus (CCHFV), which is a member of the Nairovirus genus within the Bunyaviridae family. This family comprises more than 300 species grouped into five distinct genera. Members of the Bunyaviridae family are enveloped, single-stranded RNA genomes of negative polarity.5 CCHFV has the most extensive geographic distribution among all tick-borne viruses and is widespread in Eurasia (including the Middle East) and Africa.6 In Iraq, several cases were reported in 1979 with a high mortality rate, and all such individuals were in direct contact with domestic animals.7,8,9 Infection with CCHFV in humans occurs through tick bites or direct contact with blood or tissues of infected livestock and patients. Workers in the livestock and agricultural industries and slaughterhouses, medical laboratory staff, and health care workers are at high risk.3 The virus was first isolated from ticks of the genus Hyalommaduring the 1960s. It has been detected in at least 31 species of ticks in the Ixodidae and Argasidae families.10 A common pathogenic feature of CCHFV is its ability to disable host immune response.11 The main pathological mechanism is believed to be vascular endothelial damage and the main target is thought to be the reticuloendothelial system.12
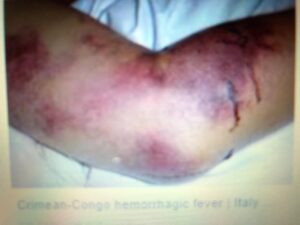
In addition to its effects on animals, CCHFV is a main pathogen for humans. The most commonly observed symptoms and clinical signs in humans are fever, severe headache, dizziness, photophobia, myalgia, arthralgia, bleeding, ecchymosis, hematemesis, melena, epistaxis, haematuria, heamoptysis, nausea, and vomiting.13 Early diagnosis of the virus is crucial to preventing and controlling further spreading and to seeking treatment. Several methods are used for the diagnosis of CCHF such as Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR),14 Enzyme-Linked Immunosorbent Assay (ELISA),15 and viral isolation.16 The control and prevention of this viral disease requires controlling ticks,17 enhancing surveillance using standardized case definition, increasing laboratory capacity within already endemic areas and areas at risk for CCHF expansion, awareness of prophylactic measures in the general population and in healthcare workers, and modifying their risk for infection.18,19 Vaccines for CCHFV are not available in most countries for either humans or animals.20
The aim of this study was to detect CCHFV in human, cattle and ticks through molecular and serological detection methods. The research was conducted in Sulaimani province of Kurdistan region, Iraq.
- Materials and Methods
| *Special description of the title.(dispensable) |
2.1. Study Area and Sample Collection: Sera collection was conducted between May 2014 and February 2015. The study was confined to three villages (Kaochktash, Kanipanka, and Darashesh) in the Sharazoor district around Sulaimani province (Figure 1), where cases of CCHF were


Figure 1.Study area: Sharazoor district of Sulaimani province.
Random blood samples were collected from human and cattle. This represents about 1% of the targeted
Population of human and cattle.
Ninety-four human blood samples were collected and divided into two parts, the first for virus detection by RT-PCR and the second for detection of the anti-CCHFV antibody by ELISA. 110 blood samples were collected from cattle for virus detection by RT-PCR. A total of 55 ticks were also collected from cattle by special forceps into test tubes, then transported to the research laboratory for virus detection by RT-PCR. Sixteen serum samples were collected from the occupational risk group (butchers at slaughterhouses who were in contact with animals). As controls, 30 serum samples were collected from persons living in Sulaimani city center area who had never been in contact with animals.
2.2. Nested RT-PCR for CCHFV Detection:
The extraction of viral RNA from human blood samples, cattle blood samples, and ticks was performed according to manufacturer procedures (the Geneaid Company, South Korea). Oligonucleotides were ordered from the Oligo (MACROGEN) Company, South Korea (Table 1).
Table 1.Oligonucleatides (primers) for RT – PCR for the detection of Genes of CCHFV. F: Forward; R:Reverse
| Gene | Polarity of Primer | Primer Sequence | Amplicon Size bp | Primer Design |
| CCHF Segment S gene
CCHF Segment S gene
Beta actin mRNA
Glycerald ehyde-3- phosphate dehydrogenase mRNA
| F | CTG CTC TGG TGG AGG CAA CAA | 452 | NCBI |
| R | TGG GTT GAA GGC CAT GAT T | |||
| F | AGG TTT CCG TGT CAA TGC AAA | 207 | NCBI | |
| R | TTG ACA AAC TCC CTG CAC CAG T | |||
| F | ATG TGT GAC GAC GAG GTT GCC GC | 432 | NCBI | |
| R | GTA CAG CGA CAG CAC GGC CTG G | |||
| F | CCA CTC CCA ACG TGT CTG TT | 452 | NCBI | |
| R | TGA TGG TAC ACA AGG CAG GG |
2.3. Detection of Anti-CCHFV Antibody:The ELISA kit for detection of human anti-CCHFV IgG antibody was performed according to the manufacturer’s procedures (Alpha Diagnostic International, Texas, USA).
- Statistical Analysis: The Statistical Package for Social Science (SPSS) version 21 was used for analysis.
- Results
4.1. Detection of CCHFV from Human and Animal Samples Using RT-PCR:
RT-PCR was used for the detection of CCHFV-RNA from blood samples collected from humans and cattle in the three villages; the results were negative for all 94 human and 110 cattle samples. The same technique was used to detect viral RNA from tissues prepared from 55 pooled ticks collected from cattle in the same area, and all were negative (Table 2). To confirm the accuracy of the results of the RT-PCR, two housekeeping genes (bovine and tick) were used and the result was negative.
Table 2.The results of RT – PCR from human, cattle and tick samples.
| Samples | No. of Samples | Positive Cases No. (%) | Negative Cases No. (%) | |
| Human | 94 | 0(0.0) | 94(100.0) | |
| Cattle | 110 | 0(0.0) | 110(100.0) | |
| Tick | 55 | 0(0.0) | 55(100.0) | |
4.2. Seroprevalence of IgG Anti-CCHFV Antibody in Human Sera:
ELISA was used on negative human serum samples by RT-PCR to determine the immunological status of the population in this study who have daily contact with cattle or who are members of the risk group (butchers from Sulaimani slaughterhouse), and a control group population of 30 young students (aged 20-30) from Sulaimani university who were never in contact with cattle, according to the data collection sheet. The overall anti-CCHFV IgG antibody prevalence among the residents of the three villages was 46.87% (30/64). The sex prevalence for females was 36.7% (11/30) and 63.3% (19/30) for males; no significant statistical differences were found between males and females (p= 0.515). All 30 serum samples from young students were seronegative for the CCHFV IgG antibody, while serum samples obtained from butchers revealed anti-CCHFV IgG prevalence of 18.75% (3/16). Seroprevalence of the anti-CCHFV IgG antibody was 47.36% (9/19), 66.66% (10/15) and 57.14% (8/14) in farmers who had daily contact with cattle in Kaochktash, Kanipanka, and Darashesh villages, respectively (Table 3). There was a significant difference between risk groups and the control group (p=0.001).
Table 3.Seroprevalence of IgG anti-CCHFV antibody from human sera.
| Location | No. of Samples | Positive Cases No. (%) | Negative Cases No. (%) | P value | |
| KaochktashVillage | 19 | 9 (47.36) | 10 (52.63) | 0.001* | |
| Kanipanka Village | 15 | 10 (66.66) | 5 (33.3) | ||
| Darashesh Village | 14 | 8 (57.14) | 6 (42.85) | ||
| Slaughterhouse | 16 | 3 (18.75) | 13 (81.25) | ||
| City Center | 30 | 0 (0.0) | 30 (100.0) | ||
| Total | 94 | 30 (31.91) | 64 (68.0) | ||
* Chi-square test (chi = 29.86, df = 4, p< 0.01), significant differences.
4.3. The Relationship between Age Groups and the Prevalence of the Anti-CCHFV Antibody:
The highest prevalence was shown among those aged 30-40 (56.25%), and the lowest was among the 40-50 year and 50-60 year age groups (43.75%), while those aged 20-30 were seronegative for the IgG antibody (p=0.001) (Table 4).
Table 4.Age distribution in relation to serum anti-CCHFV antibodies.
| Age group | No. of samples | Positive cases No. (%) | Negative cases No. (%) | P value | |
| 20 – 30 | 30 | 0 (0.0) | 20 (100.0) | 0.001* | |
| 30 – 40 | 16 | 9 (56.25) | 7 (43.75) | ||
| 40 – 50 | 32 | 14 (43.75) | 18 (56.25) | ||
| 50 – 60 | 16 | 7 (43.75) | 9 (56.25) | ||
| Total | 94 | 30 (31.91) | 64 (68) | ||
*Chi-square test (chi = 12.55, df = 3, p< 0.01), highly significant.
- Discussion
The apparent increase in the global distribution of CCHFV coupled with circulation in numerous vertebrate species and a significant increase in case fatality rates associated with nosocomial transmission of CCHFV highlight the importance of a rapid, robust, and reliable diagnostic procedure to confirm the presence of the virus. Assays that lead to early diagnosis of CCHF will result in better prevention, patient management, isolation, and infection control strategies.21 Serological and epidemiological surveys usually determine the endemicity of viral diseases, including CCHF, in a particular region. In endemic areas, assessment of human and livestock antibodies for CCHFV appear to be one of the best indicators of CCHF risk. CCHF is endemic in different parts of Iraq.9 Standardized diagnostic methods, increasing public awareness through health education programs, and enhancing the capacity of health and veterinary professionals should be identified as priorities in endemic areas like Iraq. Good administrative arrangement and ensuring maximum cooperation between different authorities, particularly between health and veterinary authorities are also essential.
The virus is transmitted by various tick species, but mainly those of the genus Hyalomma. Domestic ruminants are infected through tick bites, and can infect more ticks to perpetuate the virus in nature.22, 23 In rural areas in the Kurdistan region of Iraq, agriculture, animal husbandry, free grazing, uncontrolled trade, movement of sheep and goats (especially in border areas), poor farm hygiene, and lack of education and knowledge are among the factors contributing to the spread and transmission of the infection and challenge its complete eradication.
As shown in Table 2, the RT-PCR results for human, cattle, and tick samples were negative for the CCHFV genome. To confirm the reliability of RT-PCR in our study, two housekeeping genes were used to analyze the relative expression of different genes for gene expression analysis and as indicators of perfect nucleic acid extraction, quality of samples and quality of PCR. The results were positive for both housekeeping genes and were negative for all samples tested. This diminishes the possibility of false negative results. The selection of specific viral primers made the technique more specific. The primers were chosen from highly conserved fragments of the extreme terminal regions of the S-segment, which is more likely expected to detect all strains of the virus. It is well documented that the CCHFV and RNA S-segment, which codes for viral nucleoprotein (NP) and non-structural proteins, has less variable nucleotide sequences among cognate genes of CCHFV strains.24
One of the most striking questions about the geographical distribution of CCHFV is the lack of clinical cases in the Western Mediterranean, west of the main distribution area of the pathogen. This is of special interest because the main tick vector Hyalommamarginatum is common and abundant in places like Spain and Southern Italy, as well as in Mediterranean and African countries where neither the disease nor the virus have been reported.25 Thirty-six years ago, CCHF was endemic in the study area where clinical cases were reported, but since 1997 there have been no reports of human infection cases of the disease in Sulaimani province, according to data from the directorate of health and protection in Sulaimani province. It would appear that CCHF is not currently a serious health problem in this province. In this study, molecular analysis showed that ticks collected from the three villages were CCHFV-negative, which was reflected in the negative results in human and cattle samples. The absence of infection may be in part due to better eradication of ticks, which are the main vector and reservoir for the virus. The regular implementation of dipping and the use of acaricide on livestock have greatly reduced the vector population and the reservoir for the CCHFV, and are interrupting the life cycle of ticks and the virus.
In this paper, the prevalence of the anti-CCHFV IgG antibody, which is indicative of previous exposure to the virus, was 46.87%. The failure of the public health system to detect acute cases may be attributable to the non-specific nature of the virus’ clinical presentation, which makes it difficult to differentiate CCHF from other endemic cases of febrile illness. Strains of CCHFV like AP92 are considered to cause a much milder form of human disease and may represent a predominantly moderate severity of infection.26,27
As shown in Table 4, the age group of 30-40 years had the highest (56.52%) seroprevalence rate. This finding is comparable to findings reported in Iran and Kenya,28,29and confirms the important risk factors for CCHFV exposure, which include those in high risk occupations (farmers and butchers) where individuals have contact with livestock, and those over the age of 30. In this study, comparing the seroprevalence of risk groups that had daily contact with livestock (46.87%) to the control group (0%), who are young students living in urban areas, showed that there are highly significant differences between them (p<0.01). This finding corresponds with findings observed in Kenya and South Africa.29, 30 It is consistent with studies carried out in Africa, Europe and the Middle East, which showed that people working with livestock, such as farmers and butchers, have the highest prevalence of anti-CCHFV IgG antibody, indicating their high risk of infection.28, 31 Despite reports of the disease from the neighboring countries of Iran (mostly from areas closer to the Pakistani border), Russia, and Bulgaria, the number of reported cases in these countries is much higher than in Iraq.32 It is unclear in the evidence when and how seropositive humans in the present study have been infected without any course of disease or clinical symptoms. However, they might have been in contact with infected ticks or cattle during their early lives (before 1997), become subclinically infected, and seropositive thereafter.
- Conclusion
According to the data and findings presented in this study, Sulaimani province has become an area in which CCHF is not currently a serious health problem. However, more efforts should be made by health and veterinary authorities in this province to increase public awareness of the disease and to prepare to properly deal with any cases in the future. These recommendations can be achieved successfully with multidisciplinary, integrated, and collaborative approaches.
Acknowledgements
Thanks to the Department of Medical Microbiology, School of Medicine, Sulaimani University for their cooperation and support.
References
- Mardani, M; and Jahromi, M.K. (2007). Crimean-Congo Hemorrhagic Fever. Archives of Iranian Medicine; 10 (2): 204-214.
- World Organization for Animal Health (WHO), 2016: OIE-Listed disease, infections and infestations in force in 2016. Available at: http://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2016/
- Faghihi , F.; Chinikar, S.;Telmadarraiy, Z.;et al. (2015). Crimean-congo hemorrhagic fever: A seroepidemiological and molecular survey in north of Iran. Journal of Entomology and Zoology Studies; 3 (1): 156-159
- Chinikar, S; Moghadam A. H.; ParizadehSM. J.; et al. (2012).Seroepidemiology of Crimean Congo Hemorrhagic Fever in Slaughterhouse Workers in North Eastern Iran. Iran J Public Health; 41 (11): 72-77.
- Clerex-van Haaster, C.M.; Clerex, J.P.; Ushijima, H.; Akashi, H.; Fuller, F.; Bishop, D.H. (1982). The 3 terminal RNA sequences of bunyaviruses and nairoviruses (Bunyaviridae): evidence of end sequence generic differences within the virus family. J Gen Virol; 61:33-34.
- Maltezou, H.C.; and Papa, A. (2011). Crimean-Congo hemorrhagic fever: epidemiological trends and controversies in treatment. BMC Medicine; 9: 131.
- Al-Tikriti, S.K.; Al-Ani, F.; Jurji, F.J.; Tantawi, H.; Al-Mosleh, M.; Al-Janabi, N.; et al. (1981). Congo-Crimean hemorrhagic fever in Iraq. Bulletin of the World Health Organization; 59(1).
- Tantawi, H.H.; Al-Mosleh, M.I.; Al-Janabi, N.Y.; Al-Bana, A.S.; Mahmud, M.I.; Jurji, F.; Yonan, M.S.; Al-Ani, F.; Al-Tikriti, S.K.(1980). Crimean-Congo hemorrhagic fever virus in Iraq: isolation, identification and electron microscopy. ActaVirol (Praha).; 24:464-467.
- Emad, S.A.; Nabeel, A.M.; Sumad, M.W. (2012). Crimean-Congo hemorrhagic fever in Iraq during 2010. Proceeding of the Eleventh Veterinary Scientific Conference; pp:99-103.
- Kayedi, M. H.; Chinikar, S.; Mostafavi, E.; et al. (2015). Crimean–Congo Hemorrhagic Fever Virus Clade IV (Asia 1) in Ticks of Western Iran. J Med Entomol ;52(5):1144-1149.
- Bereczky, S.; Lindegren, G.; Karlberg, H.; Akerstrom, S.; Klingstrom, J.; Mirazimi, A.; (2010). Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J. Gen. Virol.; 91:1473-1477.
- Marty, A.M.; Jahrling, P.B.; Geistbert, T.W.; (2006). Viral hemorrhagic fevers. Clin Lab Med.; 26:345-386, viii.
- Baraiteanu, S.; Danes, D.; Dumitrescu, G. V.; Ionescu, L. E.; Vladimirescu, A. F. (2015). MOLECULAR BIOLOGY TECHNIQUES USED IN ACTIVE SURVEILLANCE OF CRIMEAN–CONGO HAEMORRHAGIC FEVER IN RUMINANTS: A CRITICAL REVIEW. Scientific Bulletin. Series F. Biotechnologies, Vol. XIX, ISSN 2285-1364: 93-96.
- Duh, D.; Saksida, A.; Petrovec, M.; Dedushaj, I.; Avsic-Zupanc, T.; (2006). Novel one step real-time RT-PCR assay for rapid and specific diagnosis of Crimean-Congo hemorrhagic fever encountered in the Balkans. J. Virol. Methods; 133:175-179.
- Yapar, M.; Aydogan, H.; Pahsa, A.; Besirbellioglu, B.A.; Bodur, H.; Basustaoglu, A.C.; (2005). Rapid and quantitative detection of Crimean-Congo hemorrhagic fever virus by one step real-time RT-PCR. Jpn J Infect Dis.; 58:358-362.
- Shephered, A.J.; Swanepoel, R.; Leman, P.A.; Shephered, S.P. (1986). Comparison of methods for isolation and titration of Crimean- Congo hemorrhagic fever virus. J. Clin. Microbiol.; 24:654-656.
- Keshtkar-Jahromi, M.; Kuhn, JH.;Christova, I.; Bradfute, SB.; Jahrling, PB.; Bavari, S. (2011). Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antiviral Res. 90(2): 85-92.
- Maltezou, H.C.; Andonova, L.; Andraghetti, R.; Bouloy, M.; Ergonul, O.; Jongejan, F.; Kalvatchev, N.; Nichol, S.; Niedrig, M.; Platonov, A.; Thomson, G.; Leitmeyer, K.; and Zeller, H. (2010). Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Eurosurveillance, 15(10): 1-4.
- Bajpai, S.; and Nadkar, M.Y. (2011). Crimean-Congo hemorrhagic fever: requires vigilance and not panic. J Assoc Physicians India; 59: 164-167.
- Papa, A.; Papadimitriou, E.; and Christova, I. (2011a). The Bulgarian vaccine Crimean-Congo hemorrhagic fever virus strain. Scand J Infect Dis; 43: 225-229.
- Atkinson, B.; Chamberlain, J.; Logue, C.H.; Cook, N.; Bruce, C.; Dowall, S.D.; and Hewson, R. (2012). Development of a real-time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector Borne Zoonotic Dis. 12:786-793.
- Hoogstraal, H. (1979). The epidemiology of tick-borne Crimean-Congo hemorrhagic fever virus in Asia, Europe, and Africa. J. Med. Entomol.; 15:307-417.
- Whitehouse, C.A. (2004). Crimean-Congo hemorrhagic fever . Antiviral Res.; 64:145-160.
- Deyde, V.M.; Khristova, M.L.; Rollin, P.E.; Ksiazek, T.G.; Nichol, S.T.; (2006). Crimean-Congo hemorrhagic fever virus genomics and global diversity. J. Virol.; 80(17): 8834-8842.
- Estrada-Pen, A.; Lisa, J.; Jolyon, M.; Zati, V.; and Farida, T.; (2012). Unraveling the ecological Complexities of Tick-Associated Crimean-Congo Hemorrhagic Fever Virus Transmission: A gap analysis for the western palearctic. Vectoe-borne and Zoonotic Diseases; 12(9): 743-752.
- Antoniadis, A.; and Casals, J.; (1982). Serological evidence of human infection with Congo-Crimean hemorrhagic fever virus in Greece. Am J Trop Med Hyg.;31: 1066-1067.
- Ozkaya, E.; Dincer, E.; Carhan, A.; Uyar, Y.; Ertek, M.; Whitehouse, C.A.; (2010). Molecular epidemiology of Crimean-Congo hemorrhagic fever virus in Turkey: occurrence of local topotype. Virus Res.; 149: 64-70.
- Izadi, S.; Holakouie-Naieni, K.; Madjdzadeh, S.R.; (2004). Crimean-Congo hemorrhagic fever in Sistan and BalouchestanProvince of Iran: a case control study on epidemiological characteristics. Int J Infect Dis.; 8: 299-306.
- Olivia, W.L.; Zephania, I.; Caroline, T.; Edith, C.; Benedict, O.; Lillian, M.; Marietjie, V.; Anne, F.; and Rosemary, S.; (2012). Seroprevalence of Crimean-Congo hemorrhagic fever virus in Ijara District, Kenya. Vector Borne Zoonotic Dis.; 12(9): 727-732.
- Fisher-Hoch, S.P.; McCormick, J.B.; Swenpopel, R.; (1992). Risk of human infection with Crimean-Congo hemorrhagic fever virus in South Africa rural community. Am J Trop Med Hyg.; 47: 337-345.
- Heymann, D.L.; Washington, D.C. (2004). American Public Health Association; 35-37.
- Alavi-Naini, R.; Moghtaderi, A.; Koohpayeh, H.R.; (2006). Crimean-Congo hemorrhagic fever in southeast of Iran. J Infect.; 52: 378-382.